Attribution: The post is a guide that was originally published on the North Carolina Public Health website but has since been taken offline. It is a very thorough guide covering everything you need to know to efficiently maintain the chemicals in your pool. The original article was written with US Imperial units which have been converted to metric.
Contents
Proper water chemistry is essential to maintaining safe and consistent swimming pool operation. Chemicals used in swimming pools include: Disinfectants to destroy harmful or otherwise objectionable organisms; Alkalinity and pH Adjusters to maintain a consistent acid-base relationship and acid buffering capacity; Chlorine Stabilizer to prevent unnecessary loss of chlorine; Algicide to kill and prevent algae, and Filter Aids to help remove foreign material. Following is a discussion of various factors which affect water chemistry, how they affect swimming pools and how to use pool chemicals to restore a properly balanced water chemistry.
pH
pH is the single most important element in swimming pool water chemistry. It affects every other chemical balance in pool water.
pH is a measure of hydrogen ion (H+) concentration in water. It indicates the relative acidity or basicity of pool water. pH is measured on a scale of 0 (strong acid) to 14 (strong base) with 7 being the neutral pH.
In pools a slightly alkaline pH of 7.4 to 7.6 is most desirable because this range is most comfortable to the human eye and provides for optimum use of free chlorine while maintaining water that is not corrosive or scale forming.
If pH is too low (below 7)
- Water becomes acidic
- Chlorine residuals dissipate rapidly
- Eye irritation occurs
- Plaster walls are etched
- Metal fittings, pump impeller, heater core may corrode
- Dissolved metals may leave stains on walls
- Rapid Loss of alkalinity
If pH is too high (above 8)
- Chlorine activity is slowed and inefficient
- Scale formation and discoloration of pool walls
- Water becomes cloudy
- Filter is overworked
- Eye irritation may occur
pH Adjustment
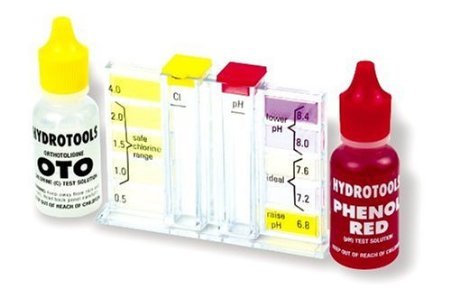
To avoid the problems listed above, pH must be maintained between 7.2 and 7.8. The most desirable level for pH is between 7.4 and 7.6. If pH is too low – run alkali demand test if available. Raise pH by adding soda ash (sodium carbonate). Never add more than 2 lbs per 10,000 gallons in a single treatment. Be sure the pump is running when chemicals are added. Allow to recirculate then retest to determine if further treatment is necessary. Caustic soda (sodium hydroxide) is sometimes used with chemical feed pumps to raise pH. If problems with low pH persist, it may be necessary to raise total alkalinity to stabilize the pH.
Chart No. 1 – Raising pH with Soda Ash
[tab]
[tab_item title=”Metric”]
| Litres in Pool | |||||||
|---|---|---|---|---|---|---|---|
| pH | 4,000 | 20,000 | 40,000 | 60,000 | 80,000 | 100,000 | 200,000 |
| 7.2-7.4 | 20 g | 100 g | 200 g | 300 g | 400 g | 500 g | 1000 g |
| 7.0-7.2 | 25 g | 125 g | 250 g | 375 g | 500 g | 625 g | 1250 g |
| 6.6-7.0 | 40 g | 200 g | 400 g | 600 g | 800 g | 1000 g | 2000 g |
| Under 6.7 | 50 g | 250 g | 500 g | 750 g | 1000 g | 1250 g | 2500 g |
[/tab_item]
[tab_item title=”Imperial”]
| Gallons in Pool | |||||||
|---|---|---|---|---|---|---|---|
| pH | 1,000 | 5,000 | 10,000 | 15,000 | 20,000 | 25,000 | 50,000 |
| 7.2-7.4 | 2/3 oz. | 3 oz. | 6 oz. | 9 oz. | 12 oz. | 1 lb. | 2 lbs. |
| 7.0-7.2 | 3/4 oz. | 4 oz. | 8 oz. | 12 oz. | 1 lb. | 1 1/4 lbs. | 2 1/2 lbs. |
| 6.6-7.0 | 1 1/4 oz. | 6 oz. | 12 oz. | 1 lb. | 1 1/2 lbs. | 2 lbs. | 4 lbs. |
| Under 6.7 | 1 1/2 oz. | 8 oz. | 1 lb. | 1 1/2 lbs. | 2 lbs. | 2 1/2 lbs | 5 lbs |
[/tab_item]
[/tab]
If pH is too high – run acid demand test if available. pH is lowered by adding muriatic acid (hydrochloric acid) or sodium bisulfate. Carefully add acid at the deep end of the pool. Try not to pour acid near pool walls or fittings. Remember: When using or diluting acids,” do as you oughta, add the acid to the water” (never add water to acid)
NOTE: 10 lbs. sodium bisulfate is roughly the same as 1 gal. muriatic acid.
Chart No. 2 – Lowering pH with Muriatic Acid
(If pH is over 7.6, add this amount of acid, then retest)
[tab]
[tab_item title=”Metric”]
| Litres in Pool | |||||||
|---|---|---|---|---|---|---|---|
| pH | 4,000 | 20,000 | 40,000 | 60,000 | 80,000 | 100,000 | 200,000 |
| 7.6-7.8 | 39 ml | 185 ml | 375 ml | 565 ml | 750 ml | 1 l | 2 l |
| 7.8-8.0 | 47 ml | 250 ml | 500 ml | 750 ml | 1 l | 1.25 l | 2.5 l |
| 8.0-8.4 | 80 ml | 375 ml | 750 ml | 1.25 l | 1.5 l | 2 l | 4 l |
| Over 8.4 | 95 ml | 500 ml | 1 l | 1.5 l | 2 l | 2.5 l | 5 l |
[/tab_item]
[tab_item title=”Imperial”]
| Gallons in Pool | |||||||
|---|---|---|---|---|---|---|---|
| pH | 1,000 | 5,000 | 10,000 | 15,000 | 20,000 | 25,000 | 50,000 |
| 7.6-7.8 | 1 1/4 oz. | 6 oz. | 12 oz. | 18 oz. | 24 oz. | 1 qt. | 2 qts. |
| 7.8-8.0 | 1 1/2 oz. | 8 oz. | 16 oz. | 24 oz. | 1 qt. | 1 1/4 qts. | 2 1/2 qts. |
| 8.0-8.4 | 2 1/2 oz. | 12 oz. | 24 oz. | 1 1/4 qts. | 1 1/2 qts. | 2 qts. | 1 gal. |
| Over 8.4 | 3 oz. | 16 oz. | 1 qt. | 1 1/4 qts. | 2 qts. | 2 1/2 qts. | 1 1/4 gal. |
[/tab_item] [/tab]
Factors which affect pH:
| Lowers pH | Raises pH |
|---|---|
| Acid | Soda Ash |
| Gas Chlorine | Sodium Hypochlorite |
| Trichlor Chlorine | Calcium Hypochlorite |
| Dichlor Chorine | Caustic Soda |
| Rain Water | Bicarbonate of Soda |
| Alum | Swimmer Wastes |
| Organic Litter | Algae Growth |
| Make up Water | Make up Water |
Total Alkalinity (TA)
Total alkalinity is closely associated with pH but rather than a measure of hydrogen ion concentration it is a measure of the ability of a solution to neutralize hydrogen ions. Expressed in parts per million (ppm), total alkalinity is the result of alkaline materials including carbonates, bicarbonates and hydroxides – mostly bicarbonates. This acid neutralizing (buffering) capacity of water is desirable because it helps prevent wide variations in pH whenever small amounts of acid or alkali are added to the pool. Total alkalinity is a measure of water’s resistance to change in
pH.
Total alkalinity should be maintained in the range of 80 to 150 ppm.
If total alkalinity is too low:
- pH changes rapidly when chemicals or impurities enter the water. pH may drop rapidly, causing etching and corrosion.
If total alkalinity is too high:
- pH becomes difficult to adjust.
- High pH often occurs causing other problems such as; cloudy water, decreased disinfectant effectiveness, scale formation and filter problems.
Raising total Alkalinity – Total alkalinity can be raised by the addition of bicarbonate of soda (sodium bicarbonate, baking soda). 1.4 lbs. bicarbonate of soda per 10,000 gallons will raise total alkalinity 10 ppm.
Chart No. 3 – Raising Alkalinity Using Sodium Bicarbonate
[tab]
[tab_item title=”Metric”]
| Increase | Litres in Pool | ||||||
|---|---|---|---|---|---|---|---|
| (ppm) | 4,000 | 20,000 | 40,000 | 60,000 | 80,000 | 100,000 | 200,000 |
| 10 | 0.1 kg | 0.3 kg | 0.7 kg | 1 kg | 1.3 kg | 1.7 kg | 3.4 kg |
| 20 | 0.1 kg | 0.7 kg | 1.3 kg | 2.0 kg | 2.7 kg | 3.4 kg | 6.7 kg |
| 30 | 0.2 kg | 1.0 kg | 2.0 kg | 3.0 kg | 4.0 kg | 5.0 kg | 10 kg |
| 40 | 0.3 kg | 1.3 kg | 2.7 kg | 4.0 kg | 5.4 kg | 6.7 kg | 13.4 kg |
| 50 | 0.3 kg | 1.7 kg | 3.4 kg | 5.0 kg | 6.7 kg | 8.4 kg | 16.8 kg |
[/tab_item]
[tab_item title=”Imperial”]
| Increase | Gallons in Pool | ||||||
|---|---|---|---|---|---|---|---|
| (ppm) | 1,000 | 5,000 | 10,000 | 15,000 | 20,000 | 25,000 | 50,000 |
| 10 | 0.14 lbs | 0.7 lbs. | 1.4 lbs. | 2.1 lbs. | 2.8 lbs. | 3.5 lbs. | 7 lbs. |
| 20 | 0.28 lbs. | 1.4 lbs. | 2.8 lbs. | 4.2 lbs. | 5.6 lbs. | 7.0 lbs. | 14 lbs. |
| 30 | 0.42 lbs. | 2.1 lbs. | 4.2 lbs. | 6.3 lbs. | 8.4 lbs. | 10.5 lbs. | 21 lbs. |
| 40 | 0.56 lbs. | 2.8 lbs. | 5.6 lbs. | 8.4 lbs. | 11.2 lbs. | 14.0 lbs. | 28 lbs. |
| 50 | 0.70 lbs. | 3.5 lbs. | 7 lbs. | 10.5 lbs | 14.0 lbs. | 17.5 lbs. | 35 lbs. |
[/tab_item]
[/tab]
In some cases, soda ash can be used to raise total alkalinity. Pound for pound, soda ash raises alkalinity 60 percent more than sodium bicarbonate and is cheaper than sodium bicarbiante. The problem with using soda ash to increase alkalinity is it drastically increases pH. This can cause cloudy water and scale formation. Soda ash should only be used to increase total alkalinity if you also need to increase the pH or if only small increases in alkalinity are needed.
Chart No. 4 – Raising Alkalinity Using Soda Ash
[tab]
[tab_item title=”Metric”]
| Increase | Litres in Pool | ||||||
|---|---|---|---|---|---|---|---|
| (ppm) | 4000 | 20,000 | 40,000 | 60,000 | 80,000 | 100,000 | 200,000 |
| 10 | 40 g | 210 g | 420 g | 630 g | 850 g | 1.06 kg | 2.12 kg |
| 20 | 90 g | 420 g | 850 g | 1.27 kg | 1.69 kg | 2.12 kg | 4.23 kg |
| 30 | 120 g | 630 g | 1.27 kg | 1.9 kg | 2.54 kg | 3.17 kg | 6.35 kg |
| 40 | 170 g | 850 g | 1.69 kg | 2.54 kg | 3.39 kg | 4.23 kg | 8.46 kg |
| 50 | 210 g | 1.06 kg | 2.12 kg | 3.17 kg | 4.23 kg | 5.29 kg | 10.58 kg |
[/tab_item]
[tab_item title=”Imperial”]
| Increase | GALLONS IN POOL | ||||||
|---|---|---|---|---|---|---|---|
| (ppm) | 1,000 | 5,000 | 10,000 | 15,000 | 20,000 | 25,000 | 50,000 |
| 10 | 0.09 lbs | 0.44 lbs. | 0.88 lbs. | 1.32 lbs. | 1.77 lbs. | 2.21 lbs. | 4.42 lbs. |
| 20 | 0.18 lbs. | 0.88 lbs. | 1.77 lbs. | 2.65 lbs. | 3.53 lbs. | 4.42 lbs. | 8.83 lbs. |
| 30 | 0.26 lbs. | 1.32 lbs. | 2.65 lbs. | 3.97 lbs. | 5.30 lbs. | 6.62 lbs. | 13.25 lbs. |
| 40 | 0.35 lbs. | 1.77 lbs. | 3.53 lbs. | 5.30 lbs. | 7.07 lbs. | 8.83 lbs. | 17.66 lbs. |
| 50 | 0.44 lbs. | 2.21 lbs. | 4.42 lbs. | 6.62 lbs. | 8.83 lbs. | 11.04 lbs. | 22.08 lbs. |
[/tab_item]
[/tab]
Chemical manufactures are now marketing a total alkalinity increaser which combines the effects of sodium bicarbonate and soda ash. The product sodium sesquicarbonate or sodium hydrogen carbonate affects total alkalinity more than sodium bicarbonate, but does not cause quite as much increase in pH as soda ash does.
Chart No. 5 – Raising Alkalinity Using Sodium Sesquicarbonate
[tab]
[tab_item title=”Metric”]
| Increase | Litres in Pool | ||||||
|---|---|---|---|---|---|---|---|
| (ppm) | 4,000 | 20,000 | 40,000 | 60,000 | 80,000 | 100,000 | 200,000 |
| 10 | 60 g | 300 g | 600 g | 900 g | 1.2 kg | 1.5 kg | 3 kg |
| 20 | 120 g | 600 g | 1.2 kg | 1.8 kg | 2.4 kg | 3 kg | 6 kg |
| 30 | 180 g | 900 g | 1.8 kg | 2.7 kg | 3.6 kg | 4.5 kg | 9 kg |
| 40 | 240 g | 1.2 kg | 2.4 kg | 3.6 kg | 4.8 kg | 6 kg | 12 kg |
| 50 | 300 g | 1.5 kg | 3 kg | 4.5 kg | 6 kg | 7.5 kg | 15 kg |
[/tab_item]
[tab_item title=”Imperial”]
| Increase | Gallons in Pool | ||||||
|---|---|---|---|---|---|---|---|
| (ppm) | 1,000 | 5,000 | 10,000 | 15,000 | 20,000 | 25,000 | 50,000 |
| 10 | 0.13 lbs. | 0.63 lbs. | 1.25 lbs. | 1.88 lbs. | 2.50 lbs. | 3.13 lbs. | 6.25 lbs. |
| 20 | 0.25 lbs. | 1.25 lbs. | 2.50 lbs. | 3.75 lbs. | 5.00 lbs. | 6.25 lbs. | 12.50 lbs. |
| 30 | 0.38 lbs. | 1.88 lbs. | 3.75 lbs. | 5.63 lbs. | 7.50 lbs. | 9.38 lbs. | 18.75 lbs. |
| 40 | 0.50 lbs. | 2.50 lbs. | 5.00 lbs. | 7.50 lbs. | 10.00 lbs. | 12.50 lbs. | 25.00 lbs. |
| 50 | 0.63 lbs. | 3.13 lbs. | 6.25 lbs. | 9.38 lbs. | 12.50 lbs. | 15.63 lbs. | 31.25 lbs. |
[/tab_item]
[/tab]
Lowering total Alkalinity – Total alkalinity can be lowered by adding muriatic acid or sodium bisulfate. Acid may be added in doses of up to 1 quart per 10,000 gallons. Total alkalinity tests and further required additions of acid can be made every 2 hours.
Chart No. 6 – Lowering Alkalinity Using Muriatic Acid
[tab]
[tab_item title=”Metric”]
| Decrease | Litres in Pool | ||||||
|---|---|---|---|---|---|---|---|
| (ppm) | 4,000 | 20,000 | 40,000 | 60,000 | 80,000 | 100,000 | 200,000 |
| 10 | 80 ml | 800 ml | 800 ml | 1.2 l | 1.6 l | 2 l | 4 l |
| 20 | 160 ml | 1.6 l | 1.6 l | 2.4 l | 3.2 l | 4 l | 8 l |
| 30 | 240 ml | 1.2 l | 2.4 l | 3.6 l | 4.8 l | 6 l | 12 l |
| 40 | 320 ml | 1.6 l | 3.2 l | 4.8 l | 6.4 l | 8 l | 16 l |
| 50 | 400 ml | 2 l | 4 l | 6 l | 8 l | 10 l | 20 l |
[/tab_item]
[tab_item title=”Imperial”]
| Decrease | GALLONS IN POOL | ||||||
|---|---|---|---|---|---|---|---|
| (ppm) | 1,000 | 5,000 | 10,000 | 15,000 | 20,000 | 25,000 | 50,000 |
| 10 | 2.56 oz. | 0.8 pts. | 0.8 qts. | 1.2 qts. | 1.6 qts. | 2.0 qts. | 1 gal. |
| 20 | 5.12 oz. | 1.60 pts. | 1.6 qts. | 2.4 qts. | 3.2 qts. | 1.0 gal. | 2 gal. |
| 30 | 7.68 oz. | 1.2 qts. | 2.4 qts. | 3.6 qts. | 1.2 gal. | 1.5 gal. | 3 gal. |
| 40 | 10.24 oz. | 1.6 qts. | 3.2 qts. | 1.2 gal. | 1.6 gal. | 2.0 gal. | 4 gal. |
| 50 | 12.80 oz. | 2.0 qts. | 1.0 gal. | 1.5 gal. | 2.0 gal. | 2.5 gal. | 5 gal. |
[/tab_item]
[/tab]
Chart No. 7 – Lowering Alkalinity Using Sodium Bisulfate
[tab]
[tab_item title=”Metric”]
| Decrease | Litres in Pool | ||||||
|---|---|---|---|---|---|---|---|
| (ppm) | 4,000 | 20,000 | 40,000 | 60,000 | 80,000 | 100,000 | 200,000 |
| 10 | 100 g | 500 g | 1 kg | 1.5 kg | 2 kg | 2.5 kg | 5 kg |
| 20 | 200 g | 1 kg | 2 kg | 3 kg | 4 kg | 5 kg | 10 kg |
| 30 | 300 g | 1.5 kg | 3 kg | 4.5 kg | 6 kg | 7.5 kg | 15 kg |
| 40 | 400 g | 2 kg | 4 kg | 6 kg | 8 kg | 10 kg | 20 kg |
| 50 | 500 g | 2.5 kg | 5 kg | 7.5 kg | 10 kg | 12.5 kg | 20 kg |
[/tab_item]
[tab_item title=”Imperial”]
| Decrease | Gallons in Pool | ||||||
|---|---|---|---|---|---|---|---|
| (ppm) | 1,000 | 5,000 | 10,000 | 15,000 | 20,000 | 25,000 | 50,000 |
| 10 | 0.21 lbs. | 1.06 lbs. | 2.13 lbs. | 3.19 lbs. | 4.25 lbs. | 5.31 lbs. | 10.63 lbs. |
| 20 | 0.43 lbs. | 2.13 lbs. | 4.25 lbs. | 6.38 lbs. | 8.50 lbs. | 10.63 lbs. | 21.25 lbs. |
| 30 | 0.64 lbs. | 3.19 lbs. | 6.38 lbs. | 9.56 lbs. | 12.75 lbs. | 15.94 lbs. | 31.88 lbs. |
| 40 | 0.85 lbs. | 4.25 lbs. | 8.50 lbs. | 12.75 lbs. | 17.00 lbs. | 21.25 lbs. | 42.50 lbs. |
| 50 | 1.06 lbs. | 5.31 lbs. | 10.63 lbs. | 15.94 lbs. | 21.25 lbs. | 26.56 lbs. | 53.13 lbs. |
[/tab_item]
[/tab]
Calcium Hardness
Calcium hardness is a measure of the dissolved calcium salts in water. Under normal conditions this should not be a problem in properly operated swimming pools. Estimates of the proper range of calcium hardness vary widely but the ideal level for plaster pool is generally considered to be about 250 ppm. If calcium hardness is very low then water may leach calcium from pool walls causing pitting of the plaster surface. Very high calcium hardness may contribute to scale formation and clouding of the water.
To raise calcium hardness – add calcium chloride.
Chart No. 8 – Raising Hardness With Calcium Chloride
[tab]
[tab_item title=”Metric”]
| Increase | Litres in Pool | ||||||
|---|---|---|---|---|---|---|---|
| (ppm) | 4,000 | 20,000 | 40,000 | 60,000 | 80,000 | 100,000 | 200,000 |
| 10 | 60 g | 300 g | 600 g | 850 g | 1.2 kg | 1.5 kg | 3 kg |
| 20 | 120 g | 600 g | 1.2 kg | 1.8 kg | 2.4 kg | 3 kg | 6 kg |
| 30 | 180 g | 900 g | 1.8 kg | 2.7 kg | 3.6 kg | 4.5 kg | 9 kg |
| 40 | 240 g | 1.2 kg | 2.4 kg | 3.6 kg | 4.8 kg | 6 kg | 12 kg |
| 50 | 300 g | 1.5 kg | 3 kg | 4.5 kg | 6 kg | 7.5 kg | 15 kg |
[/tab_item]
[tab_item title=”Imperial”]
| Increase | Gallons in Pool | ||||||
|---|---|---|---|---|---|---|---|
| (ppm) | 1,000 | 5,000 | 10,000 | 15,000 | 20,000 | 25,000 | 50,000 |
| 10 | 2 oz. | 10 oz. | 1 1/4 lbs. | 1 3/4 lbs. | 2 1/2 lbs. | 3 1/4 lbs. | 6 1/4 lbs. |
| 20 | 4 oz. | 1 1/4 lbs. | 2 1/2 lbs. | 3 3/4 lbs. | 5 lbs. | 6 1/4 lbs. | 12 1/2 lbs. |
| 30 | 6 oz. | 1 3/4 lbs. | 3 3/4 lbs. | 5 1/2 lbs. | 7 1/2 lbs. | 9 1/2 lbs. | 18 3/4 lbs. |
| 40 | 8 oz. | 2 1/2 lbs. | 5 lbs. | 7 1/2 lbs. | 10 lbs. | 12 1/2 lbs. | 25 lbs. |
| 50 | 10 oz. | 3 lbs. | 6 1/4 lbs. | 9 1/2 lbs. | 12 1/2 lbs. | 15 3/4 lbs. | 31 1/4 lbs. |
[/tab_item]
[/tab]
To lower calcium hardness anhydrous trisodium phosphate may be used. One pound trisodium phosphate per 10,000 gallons will lower calcium hardness 11 ppm. Use in small increments or clouding may occur. Another method of lowering calcium hardness is to simply drain off part of the
pool water and dilute the remaining water with fresh make up water.
Total Dissolved Solids (TDS)
After a pool has been in use for a time, dissolved solids may begin to accumulate. These unfilterable solids include body wastes, suntan lotion, stabilizer, chlorines, algicide, dirt, pollen, etc. Normally this is less of a problem with outdoor pools because of rain water and no use during winter months. Indoor pools sometimes have a buildup of dissolved solids requiring draining the pool and refilling with fresh water. Most pools should be drained after 3 to 5 years. Ideally pool water contains under 450 ppm total dissolved solids.
Disinfection of pool water
A proper balance of the previously described water chemistry factors will provide water that will not damage pool components and is non-irritating to swimmers. It is then necessary to provide for disinfection of the water to prevent the spread of disease organisms from person to person and prevent unwanted growth of bacteria and algae in the pool.
Chlorine
The most commonly used disinfectant for swimming pools is chlorine. In its elemental form chlorine is a heavy greenish yellow gas which is so toxic that is has been used as a weapon in chemical warfare. Because of the extremely high potential for injury or death from improper use of chlorine gas, a number of chlorine compounds have been formulated to provide chlorine in forms that can be handled and used safely by swimming pool operators.
The following forms of chlorine are commonly used in swimming pools:
Gas Chlorine: 100% available chlorine
| Advantages: | Disadvantages: |
|---|---|
| Cheapest form of chlorine | Extremely dangerous |
| No residue from carriers | Special room needed for chlorine |
| Feed equipment is expensive | |
| Special training and safety equipment needed | |
| Lowers pH, must constantly add pH increaser |
Because of the special hazards associated with the use of gaseous chlorine, its use has been prohibited at public swimming pools in North Carolina.
Calcium Hypochlorite: granular or pelletized 65% available chlorine
| Advantages: | Disadvantages: |
|---|---|
| Relatively cheap | Not stabilized – may lose strength if not tightly covered. |
| Can be mixed into solution for feed pumps | Does not dissolve completely – leaves residue |
| Can be used in some specially designed erosion feeders | Does not dissolve completely – leaves residue |
| High pH (11.7) raises pH of pool | |
| Highly reactive – may cause fires |
WARNING – do not use in closed tablet feeders designed to use other forms of chlorine
Sodium Hypochlorite: Liquid bleach 12.5% available chlorine
| Advantages: | Disadvantages: |
|---|---|
| Next to gas is the cheapest chlorine available | Bulky and heavy |
| No dissolving required – no residue | Not stabilized-loses strength rapidly |
| Can be used with chemical feed pumps | High pH (10-13) raises pH of pool |
Trichloroisocyanuric Acid: sticks or tablets 90% available chlorine
| Advantages: | Disadvantages: |
|---|---|
| Stabilized – chlorine doesn’t dissipate | Cost slightly higher |
| Easy to handle | Lowers pH – pH 2.8 |
| Low cost, low maintenance erosion feeders | Lowers total alkalinity |
| Highly concentrated – 90% available chlorine | May elevate cyanuric acid levels |
| Dissolves completely – very little residue | Not suitable for supeprchlorination |
Other forms of chlorine less commonly used are lithium hypochlorite, potassium dichloroisocyanuric acid, and sodium dichloroisocyanuric acid.
Free Chlorine Residual is the amount of chlorine in the pool which has not reacted with substances other than water. It is the chlorine which is available to disinfect pool water and oxidize organic substances. Free chlorine residual should be maintained between 1 and 3 ppm.
Combined Chlorine is chlorine in the pool which has reacted with substance other than water and is no longer available in its free state. Some combined chlorines are bactericides but they contribute little to the disinfection process. Chlorine combined with ammonia produces chloramines which cause eye irritation and an objectionable chlorine odor. For this reason combined chlorine residual should be kept to a minimum preferably below 0.2 ppm.
Total Chlorine residual is the concentration of free chlorine plus combined chlorine. To determine combined chlorine residual test for free chlorine and total chlorine.
Total chlorine – free chlorine = combined chlorine
Breakpoint Chlorination is the process by which combined chlorine and some organics are “burned out” of the pool by addition of large amounts of chlorine. The reaction of chlorine with ammonia to form chloramines occurs in several stages with free chlorine consumed at each stage. If enough chlorine is added to the water the total chlorine residual will rise to a point that forces the reaction of chlorine with ammonia to go rapidly to completion. Compounds of nitrogen and chlorine are released from the water and the apparent residual chlorine decreases. The point at which the chlorine residual suddenly drops is called the breakpoint. When enough chlorine is added to pass the breakpoint, combined chlorine compounds disappear, eye irritation potential and chlorine odors disappear, and the chlorine remaining in the water is all in the free state.
Superchlorination: In order to prevent buildup of chloramines in the pool it is necessary to periodically add large amounts of new chlorine in an effort to pass the breakpoint. Public swimming pools should be supechlorinated about once a week. The amount of chlorine needed to reach the breakpoint will vary depending on the amount of organic material introduced by bathers and on the level of free chlorine maintained in the pool. If the amount of combined chlorine is known then the amount of new chlorine needed is ten times the amount of combined chlorine. When combined chlorine residual is not known, superchlorination is accomplished by adding 10 ppm of new chlorine to the pool. Ordinarily calcium hypochlorite at a dose of at least 1 lb. per 10,000 gallons is used for superchlorination. The chart below shows the amounts of various chlorine compounds which can be used to introduce 10 ppm of chlorine to the pool.
Chart No. 9 – Superchlorination
(Amount Needed to Introduce 10 ppm)
[tab]
[tab_item title=”Metric”]
| Type of chlorine | Litres in Pool | ||||||
|---|---|---|---|---|---|---|---|
| 4,000 | 20,000 | 40,000 | 60,000 | 80,000 | 100,000 | 200,000 | |
| Sodium Hypo | 300 ml | 1.65 l | 3 l | 4.7 l | 6.3 l | 7.5 l | 15 l |
| Lithium Hypo | 115 g | 550 g | 1.05 kg | 1.6 kg | 2.15 kg | 2.7 kg | 5.4 kg |
| Dichlor | 65 g | 312 g | 600 g | 900 g | 1.2 kg | 1.5 kg | 3 kg |
| Calcium Hypo | 60 g | 300 g | 565 g | 900 g | 1.15 kg | 1.5 kg | 3 kg |
[/tab_item]
[tab_item title=”Imperial”]
| Type of chlorine | Gallons in Pool | ||||||
|---|---|---|---|---|---|---|---|
| 1,000 | 5,000 | 10,000 | 15,000 | 20,000 | 25,000 | 50,000 | |
| Sodium Hypo | 10 oz. | 1 3/4 qts. | 3 1/4 qts. | 1 1/4 gal. | 1 2/3 gal. | 2 gal. | 4 gal. |
| Lithium Hypo | 4 oz. | 1 1/4 lbs. | 2 1/3 lbs. | 3 1/2 lbs. | 4 3/4 lbs. | 6 lbs. | 12 lbs |
| Dichlor | 2 1/4 oz. | 11 oz. | 1 1/3 lbs. | 2 lbs. | 2 2/3 lbs. | 3 1/3 lbs. | 6 3/4 lbs. |
| Calcium Hypo | 2 oz. | 10 oz. | 1 1/4 lbs. | 2 lbs. | 2 1/2 lbs. | 3 1/4 lbs. | 6 1/2 lbs. |
[/tab_item]
[/tab]
Non-chlorine Shock Treatments Several products have been developed which oxidize organics without the use of chlorine. Pools which use those products can accomplish the reduction of organics without closing the pool for any longer than it takes to dissolve and distribute the chemicals. Those products are more expensive than chlorine but may be preferred where it is necessary to keep a pool open.
How pH affects free chlorine residual
Chlorine reacts with water to form Hypochlorous acid (HOC1). The reaction is different for each form of chlorine but hypochlorous acid is produced by each of those reactions and is the form in which chlorine serves best as a disinfectant. Hypochlorous acid is a weak acid and easily dissociates to an ionized hypochlorite state as shown
below.
| HOC1 Hypochlorus Acid |
increasing pH-> <- decreasing pH |
H+ Hyrdogen Ion |
+ | OC1- Hypochlorite Ion |
This is important because both hypochlorous acid and the hypochlorite ion are counted as free chlorine residual on your test kit but only the hypochlorous acid portion is an effective disinfectant. The balance between hypochlorous acid and the hypochlorite ion is affected by pH. The higher the pH, the less hypochlorous acid present and the less effective free chlorine becomes. At a pH of 7.2 about 66% of free chlorine is hypochlorous acid. At a pH of 7.8 only about 33% of free chlorine is hypochlorous acid. Thus pH control is essential for maintaining the effectiveness of chlorine as a
disinfectant.
Stabilizer – Cyanuric Acid
Hypochlorous acid is a highly unstable molecule which dissipates rapidly in the presence of sunlight. This results in considerable loss of free chlorine form pools exposed to sunlight. Proper stabilization of chlorine with cyanuric acid slows the rate of chlorine dissipation without appreciably sacrificing oxidation and disinfection activity. Reaction of free chlorine with cyanuric acid produces a form of combined chlorine (chlorimide) which is active enough to aid disinfection and show up as free chlorine residual on your test kit. Proper stabilization requires 30 to 50 ppm cyanuric acid. Outdoor pools should be initially treated with 40 ppm cyanuric acid. The chart below can be used to determine the amount of cyanuric acid needed.
Chart No. 10 – Establishing or Increasing Cyanuric Acid Level
[tab]
[tab_item title=”Metric”]
| CYA increase ppm | Litres in Pool | ||||||
|---|---|---|---|---|---|---|---|
| 4,000 | 20,000 | 40,000 | 60,000 | 80,000 | 100,000 | 200,000 | |
| 10 | 35 | 185 g | 360 g | 565 g | 750 g | 900 g | 1.8 kg |
| 20 | 70 g | 360 g | 800 g | 1.1 kg | 1.5 kg | 1.8 kg | 3.8 kg |
| 30 | 70 g | 300 g | 600 g | 900 g | 1.2 kg | 1.5 kg | 5.6 kg |
| 40 | 85 g | 300 g | 560 g | 900 g | 1.1 kg | 1.5 kg | 7.5 kg |
| 50 | 185 g | 900 g | 2.4 kg | 2.8 kg | 3.8 kg | 4.8 kg | 9.5 kg |
[/tab_item]
[tab_item title=”Imperial”]
| CYA increase ppm | Gallons in Pool | ||||||
|---|---|---|---|---|---|---|---|
| 1,000 | 5,000 | 10,000 | 15,000 | 20,000 | 25,000 | 50,000 | |
| 10 | 1 1/4 oz. | 6 1/2 oz. | 12 3/4 oz. | 1 1/4 lbs. | 1 2/3 lbs. | 2 lbs. | 4 lbs. |
| 20 | 2 1/2 oz. | 12 3/4 oz. | 1 3/4 lbs. | 2 1/2 lbs. | 3 1/3 lbs. | 4 lbs. | 8 1/3 lbs. |
| 30 | 2 1/2 oz. | 11 oz. | 1 1/3 lbs. | 2 lbs. | 2 2/3 lbs. | 3 1/3 lbs. | 12 1/2 lbs. |
| 40 | 3 oz. | 10 oz. | 1 1/4 lbs. | 2 lbs. | 2 1/2 lbs. | 3 1/4 lbs. | 16 2/3 lbs. |
| 50 | 6 1/2 oz. | 2 lbs. | 5 1/4 lbs. | 6 1/4 lbs. | 8 1/3 lbs. | 10 1/2 lbs. | 21 lbs. |
[/tab_item]
[/tab]
Cyanuric acid dissolves very slowly and is best predissolved in warm water before introducing into the pool. If it is not predissolved it may take several days to dissolve completely. Once added to the pool, cyanuric acid does not dissipate. It is removed from the pool only by splash out and backwash waste. Stabilized chlorine products such as trichloroisocyanuric acid will add stabilizer to the pool and may cause a gradual rise in cyanuric acid concentration. Excessive amounts of cyanuric acid can interfere with the disinfection process and at concentrations above 100 ppm may cause “chlorine lock” and clouding of the pool. Cyanuric acid level is lowered by draining part of the water out of the pool and diluting the remaining water with fresh water. Generally cyanuric acid level should be kept below 60 ppm. Stabilized forms of chlorine should not be used for superchlorination because cyanuric acid level may be increased.
Bromine
Bromine is chemically very similar to chlorine. Bromine compounds tend to react more slowly than chlorine compounds so bromine is generally more stable and less subject dissipation in sunlight. The dissociation of hypobromous acid into the bromine ion is less affected by pH than the corresponding reaction of chlorine. This makes bromine active over a larger range of pH than chlorine. Bromine will combine with ammonia to form bromamines similar to chlorine but unlike chloramines, bromamines are effective bactericides and do not produce the degree of odor and eye irritation associated with chloramines. Bromine is less affected by high temperature and nitrogen wastes than chlorine so it is particularly attractive for use in hot water spas. Bromine is more expensive than chlorine and has not yet received widespread acceptance by swimming pool operators.
The form of bromine most commonly used in pools and spas is the organic chemical bromo-chloro-dimethylhydantoin which contains both bromine and chlorine. It is marketed under various trade names and is generally in tablet form for use in erosion feeders..
Bromine residual should be maintained between 2 and 4 ppm. Bromine residual is measured using the DPD #1 test used to measure free chlorine. If your test kit does not include a bromine scale then bromine residual is approximately 2.25 times the reading on the chlorine scale.
Biganide disinfectants
The only disinfectant other than chlorine and bromine which has been accepted as a primary disinfectant in public swimming pools is polyhexamethylene biguanide.
Biguanide is used at a concentration of 30 to 50 parts per million and a pH of 7.2 to 7.8 to kill germs and control algae growth. A special test kit is needed to test the biguanide residual. The main advantage of biguanide is the disinfectant concentration remains fairly stable so it requires less frequent adjustment than chlorine. No automatic chemical feeder is needed.
Biguanide is not an oxidizer and will not destroy organic wastes the way chlorine and bromine do. It must be used in conjunction with a peroxide shock treatment to prevent organic wastes from accumulating in pool water. Biguanide is incompatible with chlorine and most algicides. Chlorine in make-up water can cause clouding of biguanide pools. Biguanide increases the staining potential of dissolved metals in a pool so copper based algicides, copper ion generators, and pool heaters should not be used. Only chemicals recommended by the disinfectant
manufacturers should be used.
Supplemental disinfection equipment
A variety of supplemental disinfection process equipment is being marketed for use on swimming pools. The most common are copper/silver ion generators, ozone generators, and ultraviolet light generators. While each process provides some disinfection activity, they are not accepted as primary disinfectants in public swimming pools because they are either too slow or do not provide a disinfectant residual. Supplemental disinfection equipment, if used, must be used in conjunction with a free chlorine or bromine residual.
Suggested NSPI Standards – Swimming Pools
| Minimum | Ideal | Maximum | |
|---|---|---|---|
| Free Chlorine, ppm | 1.0 | 1.0-3.0 | 3.0 |
| Combined chlorine, ppm | None | None | 0.2 |
| Bromine, ppm | 2.0 | 2.0-4.0 | 4.0 |
| pH | 7.2 | 7.4-7.6 | 7.8 |
| Total Alkalinity, ppm | 60 | 80-100 | 180 |
| (for Liquid Chlorine, Cal-Hypo and Lithium Hypo) | |||
| 100-120 | |||
| (for gas chlorine, dichlor, trichlor and bromine compounds) | |||
| TDS,ppm | 300 | 1000-2000 | 3000 |
| Calcium Hardness, ppm | 150 | 200-400 | 500-1000+ |
| Cyanuric Acid, ppm | 10 | 30-50 | 150 |
| (except where limited by Health Dept. requirements, often to 100 ppm) |
Suggested NSPI Standards – Spas
| Minimum | Ideal | Maximum | |
|---|---|---|---|
| Free Chlorine, ppm | 1.0 | 1.0-3.0 | 10.0 |
| Combined chlorine, ppm | None | None | 0.2 |
| Bromine, ppm | 2.0 | 2.0-4.0 | 10.0 |
| pH | 7.2 | 7.4-7.6 | 7.8 |
| Total Alkalinity, ppm | 60 | 80-100 | 180 |
| (for Liquid Chlorine, Cal-Hypo and Lithium Hypo) | |||
| 100-120 | |||
| (for Gas Chlorine, Dichlor,Trichlor and Bromine Compounds) | |||
| TDS,ppm | 300 | 1000-2000 | 3000 |
| Calcium Hardness, ppm | 150 | 200-400 | 500-1000+ |
| Cyanuric Acid, ppm | 10 | 30-50 | 150 |
| (except where limited by Health Dept. requirements, often to 100 ppm) |
Algae Control
Algae are tiny plants that bloom and grow in swimming pools if nutrients are present and a sufficient level of free chlorine is not maintained. Below are descriptions of the three most common algae problems in swimming pools.
Green Algae The most common algae in swimming pool floats in water and coats pool surfaces. Left unchecked green algae will very quickly turn the pool water pea green.
Mustard Algae settles on pool walls and causes a slimy yellow film.
Black Algae appears in “buds” or clumps attached to tile grout, corners, steps and pool surfaces.
Solution:
Green Algae – is very susceptible to chemical treatment. Superchlorinate with 10 to 20 ppm chlorine in the evening. Keep the filter running and brush the pool walls and bottom. Periodically check chlorine and maintain above 3 ppm until water clears. Using an algicide containing quaternary ammonia the next morning will help prevent the return of green algae.
Mustard Algae – is much more resistant to chemical treatment and clings more tightly to pool walls than green algae. Adjust pH and superchlorinate as for green algae then brush diligently. Later vacuum the pool, check chlorine and superchorinate again if necessary. Mustard algae will generally return unless treated with a special mustard algicide or a copper based algicide. Algicide should be added in the morning to treat algae in daylight – its most active period.
Black Algae – is very difficult to get rid of. It can be controlled to some extent by frequent superchlorination and diligent brushing with a stiff brush. Spot treatments can be made by turning off the recirculation pumps and pouring granular chlorine directly on recently brushed spots. Trichlor tablets can also be rubbed on recently brushed areas to spot treat. Black algae can usually be controlled with the use of strong alicides and maintenance of relatively high free chlorine residual, but complete removal of black algae may require draining and cleaning the pool.
Note: Algae blooms are a problem best avoided. Maintaining proper water quality and frequent brushing of pool walls will deprive algae of the opportunity to get started.







Respond to Water Chemistry for Swimming Pools